1) Clinical application of CD38-CAR-T cell therapy
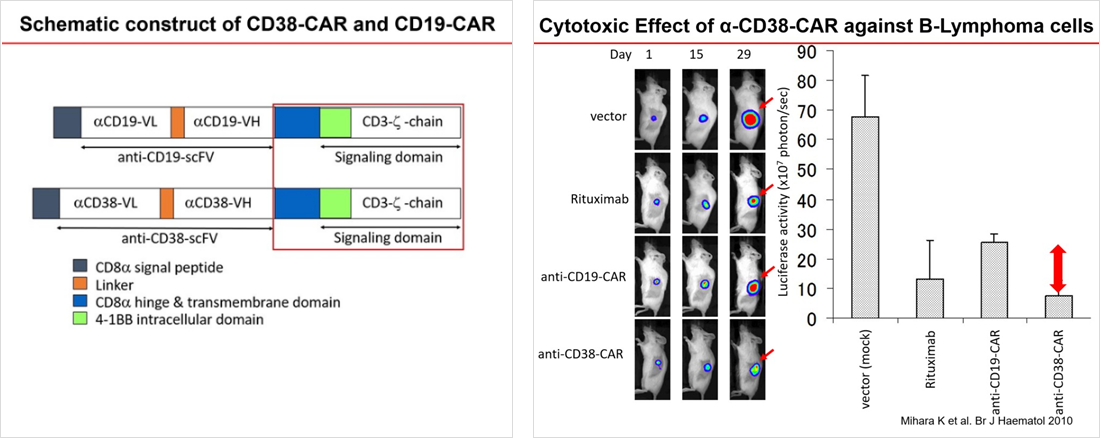
In 2004, Mihara et al. developed CD38-CAR vector based on their experience regarding research and development on Kymriah® insertion and acquired a patent through experiments. In vitro and in vivo studies showed that the CD38-CAR-T cells were effective for multiple myeloma, malignant lymphoma, acute myeloid leukemia, and adult T-cell leukemia/lymphoma. For clinical application, further research and development are being conducted.
2) Preparation of CAR backbone vectors for solid tumors
The efficacy of Tisagenlecleucel (Kymriah®) and axicabtagene ciloleucel (Yescarta®) for hematological tumors has been warranted and clinically approved for use in these tumors alone. Unfortunately, the clinical impact of CAR-T cell therapy on the effectiveness of many solid tumors has not been clearly demonstrated. So, we are preparing CAR backbone vectors for solid tumors, that is, malignant tumors with which the number of patients is the greatest.
3) Preparation of a vector that inhibits cytokine release syndrome (CRS)
CRS frequently appears during/after CAR-T cell therapy, leading to a serious condition. The administration of anti-IL-6 receptor antibody (Tocilizumab: Actemra®) has rapidly decreased the mortality rate, but 2 or 3% of patients with CRS still show a fatal outcome. When CRS involves the central nervous system, tocilizumab is basically ineffective, and a vector that inhibits CRS must be promptly developed. We are also focusing our efforts on the development of this vector.
4) Development of CAR-T cell therapy for glioblastoma
We are developing CAR-T cell therapy for glioblastoma, a highly malignant brain tumor. An extremely specific and safe vector is being prepared in parallel to the development of vectors 2) and 3).
Please feel free to contact us with any questions or concerns you may have from the following link.
Information about related facilities on campus
Kasahara Y, Shin C, Kubo N, Mihara K, Iwabuchi H, Takachi T, Imamura M, Saitoh A, Imai C. Development and characterisation of NKp44-based chimeric antigen receptors that confer T cells with NK cell-like specificity. Clin Transl Immunology 9(7):e1147 2020
Mihara K, Yoshida T, Bhattacharyya J. Basic Procedures for Detection and Cytotoxicity of Chimeric Antigen Receptors. Methods Mol Biol 1904:299-306 2019
Mihara K, Yoshida T, Takei Y, Sasaki N, Takihara Y, Kuroda J, Ichinohe T. T cells bearing anti-CD19 and/or anti-CD38 chimeric antigen receptors effectively abrogate primary double-hit lymphoma cells. J Hematol Oncol 10(1):116 2017
Yoshida T, Mihara K, Ishida S, Takei Y, Kitanaka A, Shimoda K, Morishita K, Takihara Y, Ichinohe T. All-trans retinoic acid and interferon-a increase CD38 expression on adult T-cell leukemia cells and sensitize them to T cells bearing anti-CD38 chimeric antigen receptors. Blood Cancer J 6(5):e421 2016
Yoshida T, Mihara K, Takei Y, Yanagihara K, Kubo T, Bhattacharyya J, Imai C, Mino T, Takihara Y, Ichinohe T. All-trans retinoic acid enhances cytotoxic effect of T cells with an anti-CD38 chimeric antigen receptor in acute myeloid leukemia. Clin Transl Immunology 5(12):e116 2016
Bhattacharyya J, Mihara K, Kitanaka A, Yanagihara K, Kubo T, Takei Y, Kimura A, Takihara Y. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eradicating chemotherapy-resistant B-cell lymphoma cells overexpressing survivin induced by BMI-1. Blood Cancer J 2(6):e75 2012
Mihara K, Bhattacharyya J, Kitanaka A, Yanagihara K, Kubo T, Takei Y, Asaoku H, Takihara Y, Kimura A. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eliminating myeloma cells. Leukemia 26(2):365-7 2012
Mihara K, Yanagihara K, Takigahira M, Kitanaka A, Imai C, Bhattacharyya J, Kubo T, Takei Y, Yasunaga S, Takihara Y, Kimura A. Synergistic and persistent effect of T-cell immunotherapy with anti-CD19 or anti-CD38 chimeric receptor in conjunction with rituximab on B-cell non-Hodgkin lymphoma. Br J Haematol 151(1):37-46 2010
Mihara K, Yanagihara K, Takigahira M, Imai C, Kitanaka A, Takihara Y, Kimura A. Activated T-cell-mediated immunotherapy with a chimeric receptor against CD38 in B-cell non-Hodgkin lymphoma J Immunother 32(7):737-43 2009
Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia 18(4):676-84 Leukemia 2004
![Research case 001 [Dr. Keichiro Mihara]](v7r98800000000mh-img/v7r98800000000od.jpg)