Reducing the Side Effects of Chemotherapy in Breast Cancer: DSS1 Lights a Safer Way
By modulating the level of a protein, DSS1, researchers identify a novel technique of reducing the side effects of chemotherapy in treating breast cancer
Breast cancer is the deadliest and most common type of cancer in women. The treatment options often require administration of anti-cancer drugs in high doses, as the cells develop resistance towards chemotherapy, leading to painful side effects in patients. Recently researchers have identified that reducing the level of a protein called DSS1 can increase patients’ responsiveness to chemotherapy, therefore lowering the doses of the drug as well as the chances of side effects in patients.
Globally each year, more than 600,000 women succumb to deadly breast cancer—the most common cancer affecting women. While individual or combined effects of lifestyle and environmental factors contribute to the development of breast cancer in a large percentage of the female population, the formation of malignancy is usually associated with genetic factors. For example, BRCA1 and BRCA2 are two genes that impact a person's chances of developing breast cancer. Under normal conditions, the protein products of these genes help in repairing DNA damages, thereby reducing the chances of uncontrolled cell growth and tumor development. Any “mutation” or cellular level abnormalities that hinder the functioning of the BRCA genes, thus, predispose the person to a higher chance of developing breast cancer.
As such, for decades, researchers have been focusing on deciphering the role of BRCA genes and the cellular components associated with BRCA1 and BRCA2 proteins to understand the progression of breast cancer, and design appropriate targeted therapeutics to prevent and treat the disease. Now, a group of collaborating researchers from Japan and the USA has successfully identified a protein from the BRCA-associated cellular machinery that plays a critical role in the progression of breast cancer. The prominent researchers who were part of this research project were Kazuhiko Kuwahara (Fujita Health University School of Medicine, Japan), Naomi Gondo (Division of Immunology, Aichi Cancer Center Research Institute, Nagoya, Japan), Yasuhiro Sakai (Department of Joint Research laboratory of Clinical Medicine, Fujita Health University School of Medicine, Toyoake, Japan), Zhenhuan Zhang (Radiation Oncology Department, University of Florida, Gainesville, FL), and Andri Rezano (Department of Biomedical Sciences, Division of Cell Biology, Faculty of Medicine, Universitas Padjadjaran, West Java, Indonesia). The findings of the study, recently published in Laboratory Investigation, offer important leads for developing targeted therapy for the fatal disease, too.
In their study, the researchers started by looking closely at a protein complex called TRanscription–EXport-2 (TREX-2), which is involved in the transcription and export of mRNA from the nucleus. The complex is made up of several proteins, such as GANP, PCID2, DSS1, and centrin ¾, and as per earlier reports, aberrant expression of some of these proteins leads to DNA damage that results in tumor formation. Dr. Kuwahara, the corresponding author of the study, explains what made them look into TREX-2 complex proteins, “Earlier, we observed GANP-deficiency was closely associated with breast carcinogenesis. We were therefore interested to look into other protein components of the TREX-2 complex for their possible association with breast cancer.” Based on available published information, they focused on DSS1, a protein that is known to be associated with the stabilization of the BRCA2 protein in human cell lines.
To test their assumptions, the researchers conducted a series of studies that started with checking the expression levels of various TREX-2 complex proteins, including DSS1, in breast cancer tissues, followed by cellular level experiments. They found that the DSS1 protein expression was higher in human breast carcinoma tissues than in normal tissues. In contrast, the expression of PCID2 protein was normal in malignant tissues. They also found that low DSS1 expression is associated with longer survival time in patients. Interestingly, breast cancer cells with diminished DSS1 levels were more sensitive to standard anti-cancer drugs DXR and PTX, while a high level of DSS1 made the breast cancer cells resistant to these therapeutic agents.
The findings marked a breakthrough for breast cancer treatment research. Dr. Kuwahara summarizes, “Strong side effects of anti-cancer therapeutics add to the suffering of the patients and complicates the treatment modalities. Our research suggests depleting the DSS1 protein from breast cancer cells may make the cells chemosensitive, that is more responsive to lower doses of anti-cancer drugs, which means the chances of drug-induced adverse effects in patients with breast cancer will be reduced by this technique.”
The promising result of this study generates hope for an era of safer chemotherapy, in the near future, for patients suffering from breast cancer.
Breast cancer is the deadliest and most common type of cancer in women. The treatment options often require administration of anti-cancer drugs in high doses, as the cells develop resistance towards chemotherapy, leading to painful side effects in patients. Recently researchers have identified that reducing the level of a protein called DSS1 can increase patients’ responsiveness to chemotherapy, therefore lowering the doses of the drug as well as the chances of side effects in patients.
Globally each year, more than 600,000 women succumb to deadly breast cancer—the most common cancer affecting women. While individual or combined effects of lifestyle and environmental factors contribute to the development of breast cancer in a large percentage of the female population, the formation of malignancy is usually associated with genetic factors. For example, BRCA1 and BRCA2 are two genes that impact a person's chances of developing breast cancer. Under normal conditions, the protein products of these genes help in repairing DNA damages, thereby reducing the chances of uncontrolled cell growth and tumor development. Any “mutation” or cellular level abnormalities that hinder the functioning of the BRCA genes, thus, predispose the person to a higher chance of developing breast cancer.
As such, for decades, researchers have been focusing on deciphering the role of BRCA genes and the cellular components associated with BRCA1 and BRCA2 proteins to understand the progression of breast cancer, and design appropriate targeted therapeutics to prevent and treat the disease. Now, a group of collaborating researchers from Japan and the USA has successfully identified a protein from the BRCA-associated cellular machinery that plays a critical role in the progression of breast cancer. The prominent researchers who were part of this research project were Kazuhiko Kuwahara (Fujita Health University School of Medicine, Japan), Naomi Gondo (Division of Immunology, Aichi Cancer Center Research Institute, Nagoya, Japan), Yasuhiro Sakai (Department of Joint Research laboratory of Clinical Medicine, Fujita Health University School of Medicine, Toyoake, Japan), Zhenhuan Zhang (Radiation Oncology Department, University of Florida, Gainesville, FL), and Andri Rezano (Department of Biomedical Sciences, Division of Cell Biology, Faculty of Medicine, Universitas Padjadjaran, West Java, Indonesia). The findings of the study, recently published in Laboratory Investigation, offer important leads for developing targeted therapy for the fatal disease, too.
In their study, the researchers started by looking closely at a protein complex called TRanscription–EXport-2 (TREX-2), which is involved in the transcription and export of mRNA from the nucleus. The complex is made up of several proteins, such as GANP, PCID2, DSS1, and centrin ¾, and as per earlier reports, aberrant expression of some of these proteins leads to DNA damage that results in tumor formation. Dr. Kuwahara, the corresponding author of the study, explains what made them look into TREX-2 complex proteins, “Earlier, we observed GANP-deficiency was closely associated with breast carcinogenesis. We were therefore interested to look into other protein components of the TREX-2 complex for their possible association with breast cancer.” Based on available published information, they focused on DSS1, a protein that is known to be associated with the stabilization of the BRCA2 protein in human cell lines.
To test their assumptions, the researchers conducted a series of studies that started with checking the expression levels of various TREX-2 complex proteins, including DSS1, in breast cancer tissues, followed by cellular level experiments. They found that the DSS1 protein expression was higher in human breast carcinoma tissues than in normal tissues. In contrast, the expression of PCID2 protein was normal in malignant tissues. They also found that low DSS1 expression is associated with longer survival time in patients. Interestingly, breast cancer cells with diminished DSS1 levels were more sensitive to standard anti-cancer drugs DXR and PTX, while a high level of DSS1 made the breast cancer cells resistant to these therapeutic agents.
The findings marked a breakthrough for breast cancer treatment research. Dr. Kuwahara summarizes, “Strong side effects of anti-cancer therapeutics add to the suffering of the patients and complicates the treatment modalities. Our research suggests depleting the DSS1 protein from breast cancer cells may make the cells chemosensitive, that is more responsive to lower doses of anti-cancer drugs, which means the chances of drug-induced adverse effects in patients with breast cancer will be reduced by this technique.”
The promising result of this study generates hope for an era of safer chemotherapy, in the near future, for patients suffering from breast cancer.

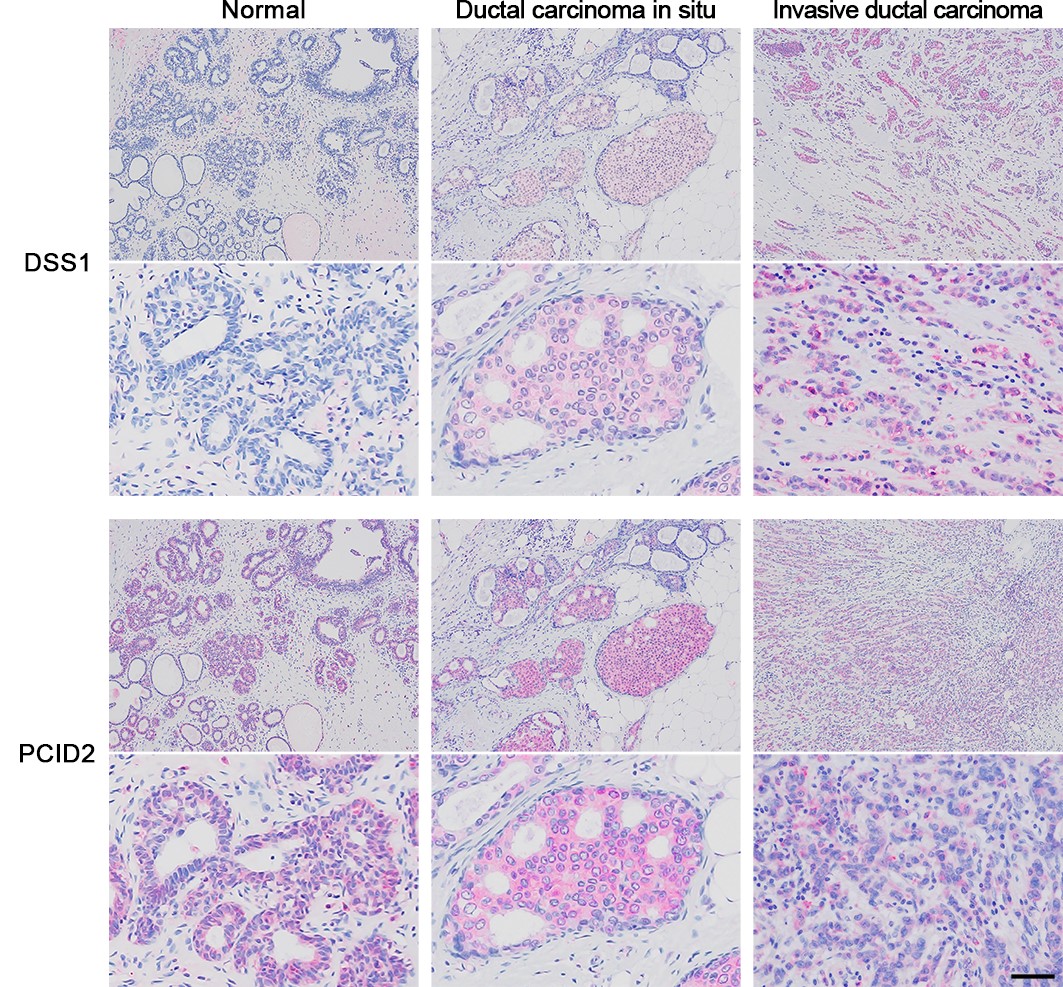
Immunohistochemistry showing DSS1 and PCID2 in normal and cancerous breast tissues
While PCID2 was always expressed, DSS1 expression was higher with increased level of malignancy.
Image courtesy: Kazuhiko Kuwahara from Fujita Health University
While PCID2 was always expressed, DSS1 expression was higher with increased level of malignancy.
Image courtesy: Kazuhiko Kuwahara from Fujita Health University
Reference
Title of original paper
Increased chemosensitivity via BRCA2-independent DNA damage in DSS1- and PCID2-depleted breast carcinomasJournal
Laboratory InvestigationDOI
10.1038/s41374-021-00613-6About Dr. Kazuhiko Kuwahara
Dr. Kazuhiko Kuwahara is a Senior Associate Professor in the Department of Diagnostic Pathology, Fujita Health University, Japan. He earned his MD degree from Saga Medical School in 1989, following which he has continued in his career as a researcher in the field of immunology. His current research focus is developing newer treatment strategies for breast cancer while reducing the side effects associated with chemotherapy. As an expert in the field, Dr. Kuwahara has published 66 research papers in reputed journals.
Funding information
This study was supported by a Grant-in-Aid for challenging Exploratory Research 16K15603 (NG), a Grant-in-Aid for Young Scientists 20K16228 (YS), and Grants-in-Aid for Scientific Research (C) 24590388, 15K10083, and 20K07686 (KK) from the Japan Society for the Promotion of Science; a Grant-in-Aid for Young Scientists from Fujita Health University (YS); and Grants-in-Aid from Aichi Cancer Research Foundation (KK), the 24th General Assembly of the Japanese Association of Medical Sciences (KK), and Aichi Health Promotion Foundation (KK). AR was supported by an Indonesian Directorate General of Higher Education (DIKTI) Scholarship.
Media contact
Kazuhiko Kuwahara
kazukuwa@fujita-hu.ac.jp
kazukuwa@fujita-hu.ac.jp
