KANPHOS: Newly Developed Database for Kinase-associated Protein Phosphorylation Eases Neural Signaling Research
Understanding neural protein phosphorylation and the role of different kinases and substrates is now made easier by the newly developed data repository, KANPHOS
Biological functions rely on accurate cellular communication involving different molecules. Most major signaling pathways involved in such communication, called signal transduction, often involve a family of kinases—enzymes catalyzing the transfer of phosphates between specific substrate molecules. A thorough understanding of kinase functionality and substrate involvement is therefore required to better understand cell communication, particularly in the brain. Scientists in Japan have now developed KANPHOS, a database that provides extensive information in this regard.
Signal transduction, or cellular communication involving chemical and physical signals transmitted by signal molecules in the extracellular and intracellular environments, leads to the generation of a signal cascade, and is essential for correct execution of biological processes. This process is facilitated by the mechanism of phosphorylation and dephosphorylation—addition or removal of phosphate groups from specific substrate molecules by a family of enzymes called kinases. Hundreds of such enzymes have been identified in humans, which play an important role in the maintenance of a variety of key functional processes. This is especially true for proper neurological functioning, with deficiencies in signaling pathways related to a variety of neurological disorders.
While understanding the components and molecular mechanisms behind these signaling strategies is important, the exact involvement of individual kinases and substrates has not been clearly elucidated. In order to bridge this gap, scientists from Fujita Health University, Toyoake, and their multi-institutional colleagues designed a database called Kinase-Associated Neural PHOspho-Signaling (KANPHOS), which provides extensive data on different aspects of phosphorylation in the brain, such as signaling strategies, phosphoproteins involved, phosphorylation sites, and participant kinases. Prof. Kozo Kaibuchi, lead researcher on the study published in Cells, explains the team’s motivation behind conducting this study, “Protein phosphorylation is crucial for revealing how organs and cells function and what happens during some diseases. Accumulating studies have identified various signaling pathways but there are thousands of proteins that can be phosphorylated, suggesting that there are possibly countless unknown signaling pathways that involve protein phosphorylation.”
This newly developed database contains information about new phosphoproteins, phosphorylation sites of interest, as well as molecular species specificity. The database is built on the software platform XOOPS module for Neuroinformatics (XooNIps), which allows for constructing a web-based database that can handle a large set of metadata in different formats. Moreover, this can be accessed worldwide, which increases the range of its possible impact.
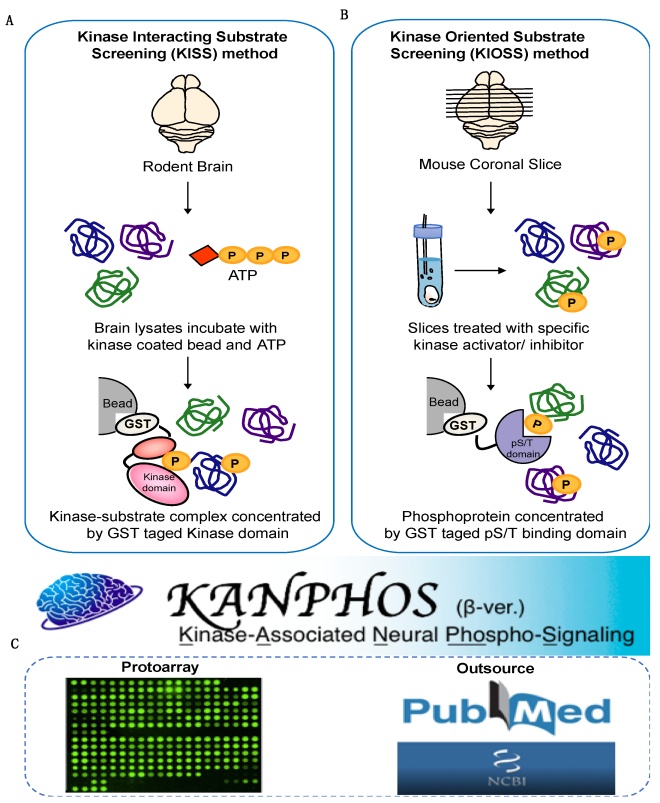
Constructing such an extensive database involved collecting information from different sources. The team manually curated existing datasets to supplement the database with information on already available phosphoproteomic data. Additionally, the researchers employed advanced techniques to further determine novel kinase-oriented protein phosphorylation information, such as the in vitro kinase interacting substrate screening method, which involved determining protein substrates through a selection process involving interactions with the catalytic (enzymatically active) domain of specific kinases, followed by their characterization with liquid chromatography/mass spectrometry (LC-MS). An in vivo technique called the kinase-oriented substrate screening method involved identifying substrates by treating tissue samples with agonists (activators) and kinase inhibitors, followed by enrichment and characterization of the identified phosphoproteins via LC-MS.
The team’s focus while designing the database was on user friendliness, besides accuracy. This was reiterated by Prof. Kaibuchi, who, speaking on behalf of co-authors Taku Nagai and Junichiro Yoshimoto from Fujita Health University, Mutsuki Amano from Nagoya University, and Takayuki Kannon from Kanazawa University, mentions, “KANPHOS is designed so that users can easily access information on genes, signaling pathways, physiological functions, and diseases associated with protein phosphorylation. This functionality will promote data-driven studies on molecular mechanisms in the brain.” Thus, the database allows for searches based on phosphorylated proteins, specific kinases, or the pathways themselves. There are also options for advanced searches, which could include searches based on gene/protein characteristics, pathways, experimental conditions, and other specific requirements. Another useful feature added is linking information from other databases directly into the webpage, ensuring a complete representation of information on a specific protein.
This database has immense potential to facilitate research in elucidating signaling pathways. The team was able to demonstrate its efficacy by exploring the different components of the adenosine A2A receptor signaling pathway mediated by adenosine—an endogenous purine nucleoside that modulates neural function—in murine brain. The database was instrumental in elucidating the specifics of the MEK-MAPK pathway driven by adenosine, and also helped in identifying a vital substrate in this process, Arhgap21. Prof. Kaibuchi is optimistic about the benefits of this database, claiming that “The information infrastructure provided by the database may help elucidate the patho-etiology and pathophysiology of neuropsychiatric disorders.”
This tool establishes the usefulness of novel information technologies in data-driven research, especially in the neurosciences, with great scope for developing therapeutic strategies for debilitating neurological disorders in future.
Japanese researchers have developed a brain protein phosphorylation database, KANPHOS
Information on phosphorylation included in the database was obtained through the kinase-
interacting substrate screening method (A), kinase-oriented substrate screening method (B), and manually curated from the literature and protoarray (C). Research utilizing the information infrastructure provided by the database is expected to lead to the elucidation of the patho-etiology and pathophysiology of neuropsychiatric disorders.
Photo courtesy: Kozo Kaibuchi from Fujita Health University
Phosphorylation in the Brain
Biological functions rely on accurate cellular communication involving different molecules. Most major signaling pathways involved in such communication, called signal transduction, often involve a family of kinases—enzymes catalyzing the transfer of phosphates between specific substrate molecules. A thorough understanding of kinase functionality and substrate involvement is therefore required to better understand cell communication, particularly in the brain. Scientists in Japan have now developed KANPHOS, a database that provides extensive information in this regard.
Signal transduction, or cellular communication involving chemical and physical signals transmitted by signal molecules in the extracellular and intracellular environments, leads to the generation of a signal cascade, and is essential for correct execution of biological processes. This process is facilitated by the mechanism of phosphorylation and dephosphorylation—addition or removal of phosphate groups from specific substrate molecules by a family of enzymes called kinases. Hundreds of such enzymes have been identified in humans, which play an important role in the maintenance of a variety of key functional processes. This is especially true for proper neurological functioning, with deficiencies in signaling pathways related to a variety of neurological disorders.
While understanding the components and molecular mechanisms behind these signaling strategies is important, the exact involvement of individual kinases and substrates has not been clearly elucidated. In order to bridge this gap, scientists from Fujita Health University, Toyoake, and their multi-institutional colleagues designed a database called Kinase-Associated Neural PHOspho-Signaling (KANPHOS), which provides extensive data on different aspects of phosphorylation in the brain, such as signaling strategies, phosphoproteins involved, phosphorylation sites, and participant kinases. Prof. Kozo Kaibuchi, lead researcher on the study published in Cells, explains the team’s motivation behind conducting this study, “Protein phosphorylation is crucial for revealing how organs and cells function and what happens during some diseases. Accumulating studies have identified various signaling pathways but there are thousands of proteins that can be phosphorylated, suggesting that there are possibly countless unknown signaling pathways that involve protein phosphorylation.”
This newly developed database contains information about new phosphoproteins, phosphorylation sites of interest, as well as molecular species specificity. The database is built on the software platform XOOPS module for Neuroinformatics (XooNIps), which allows for constructing a web-based database that can handle a large set of metadata in different formats. Moreover, this can be accessed worldwide, which increases the range of its possible impact.
Constructing such an extensive database involved collecting information from different sources. The team manually curated existing datasets to supplement the database with information on already available phosphoproteomic data. Additionally, the researchers employed advanced techniques to further determine novel kinase-oriented protein phosphorylation information, such as the in vitro kinase interacting substrate screening method, which involved determining protein substrates through a selection process involving interactions with the catalytic (enzymatically active) domain of specific kinases, followed by their characterization with liquid chromatography/mass spectrometry (LC-MS). An in vivo technique called the kinase-oriented substrate screening method involved identifying substrates by treating tissue samples with agonists (activators) and kinase inhibitors, followed by enrichment and characterization of the identified phosphoproteins via LC-MS.
The team’s focus while designing the database was on user friendliness, besides accuracy. This was reiterated by Prof. Kaibuchi, who, speaking on behalf of co-authors Taku Nagai and Junichiro Yoshimoto from Fujita Health University, Mutsuki Amano from Nagoya University, and Takayuki Kannon from Kanazawa University, mentions, “KANPHOS is designed so that users can easily access information on genes, signaling pathways, physiological functions, and diseases associated with protein phosphorylation. This functionality will promote data-driven studies on molecular mechanisms in the brain.” Thus, the database allows for searches based on phosphorylated proteins, specific kinases, or the pathways themselves. There are also options for advanced searches, which could include searches based on gene/protein characteristics, pathways, experimental conditions, and other specific requirements. Another useful feature added is linking information from other databases directly into the webpage, ensuring a complete representation of information on a specific protein.
This database has immense potential to facilitate research in elucidating signaling pathways. The team was able to demonstrate its efficacy by exploring the different components of the adenosine A2A receptor signaling pathway mediated by adenosine—an endogenous purine nucleoside that modulates neural function—in murine brain. The database was instrumental in elucidating the specifics of the MEK-MAPK pathway driven by adenosine, and also helped in identifying a vital substrate in this process, Arhgap21. Prof. Kaibuchi is optimistic about the benefits of this database, claiming that “The information infrastructure provided by the database may help elucidate the patho-etiology and pathophysiology of neuropsychiatric disorders.”
This tool establishes the usefulness of novel information technologies in data-driven research, especially in the neurosciences, with great scope for developing therapeutic strategies for debilitating neurological disorders in future.

Japanese researchers have developed a brain protein phosphorylation database, KANPHOS
Information on phosphorylation included in the database was obtained through the kinase-
interacting substrate screening method (A), kinase-oriented substrate screening method (B), and manually curated from the literature and protoarray (C). Research utilizing the information infrastructure provided by the database is expected to lead to the elucidation of the patho-etiology and pathophysiology of neuropsychiatric disorders.
Photo courtesy: Kozo Kaibuchi from Fujita Health University
Reference
Title of original paper
KANPHOS: A Database of Kinase-Associated Neural ProteinPhosphorylation in the Brain
