Challenging the Traditional Views on How the Brain Processes Movement and Sensation
Researchers unveiled novel insights into how body movements influence sensory perception, challenging long-held beliefs in neuroscience
Our body movements profoundly impact how our brain processes sensory information. Historically, it was believed that the brain's primary motor cortex played a key role in modulating sensory experiences during movement. However, a new study led by researchers from Fujita Health University has challenged this view. By selectively inhibiting different neural pathways in the mouse brain, they discovered that regions beyond the primary motor cortex significantly influence the primary sensory cortex during movement.
The brain is widely considered the most complex organ in the human body. The intricate mechanisms through which it processes sensory information and how this information affects and is affected by motor control have captivated neuroscientists for more than a century. Today, thanks to advanced laboratory tools and techniques, researchers can use animal models to solve this puzzle, especially in the mouse brain.
During the 20th century, experiments with anesthetized mice proved that sensory inputs primarily define neuronal activity in the primary sensory cortices—the brain regions processing sensory information, including touch, vision, and audition. However, over the past few decades, studies involving awake mice revealed that spontaneous behavior, such as exploratory motion and movement of the whiskers called whisking, actually regulates the activity of the sensory responses in the primary sensory cortices. In other words, sensations at the neuronal level appear substantially modulated by body movements, even though the corresponding neuronal circuits and the underlying mechanisms are not fully understood.
To address this knowledge gap, a research team from Japan investigated the primary somatosensory barrel cortex (S1)—a region of the mouse brain that handles tactile input from the whiskers. Their latest study, published in The Journal of Neuroscience on December 1, 2023, was conducted by Professor Takayuki Yamashita from Fujita Health University (FHU) and Dr. Masahiro Kawatani, affiliated with FHU and Nagoya University, along with their team.
The S1 region receives input through the axons from several other areas, including the secondary somatosensory cortex (S2), the primary motor cortex (M1), and the sensory thalamus (TLM). To investigate how these regions modulate activity in S1, the researchers turned to optogenetics (a technique for controlling activities of specific neuronal populations by light) involving eOPN3, which is a recently discovered light-sensitive protein enabling effective inhibition of specific neural pathways in response to light. Using viruses as a vector, they introduced the gene coding for this protein into the M1, S2, and TLM regions in mice. Then, they measured neural activity in S1 in awake mice performing spontaneous whisking. During this process, they selectively inhibited different signal inputs going to S1 using light as an ON/OFF switch and observed the effect at S1.
Interestingly, only signal inputs from S2 and TLM to S1, not from M1 to S1, modulated neuronal activity in S1 during spontaneous whisking. Specifically, the pathway from S2 to S1 seems to convey information about the motion state of the whiskers. Additionally, the TLM-to-S1 pathway appeared to relay information related to the phase of spontaneous whisking, which follows a repetitive and rhythmic pattern. These results challenge the established view that neuronal activity in sensory cortices is modulated primarily by motor cortices during movement, as Prof. Yamashita remarks: “Our findings provoke a reconsideration of the role of motor-sensory projections in sensorimotor integration and bring to light a new function for S2-to-S1 projections.”
A better understanding of how distinct brain regions modulate activities among each other in response to movement could lead to progress in myriad applied fields. These research insights have far-reaching implications, potentially revolutionizing fields like artificial intelligence (AI), prosthetics, and brain-computer interfaces. "Understanding these neural mechanisms could greatly enhance the development of AI systems that mimic human sensory-motor integration and aid in creating more intuitive prosthetics and interfaces for those with disabilities," Prof. Yamashita adds.
In summary, this study sheds light on the intricate workings of the brain. It also paves the way for researching the connection between body motion and sensory perception. As we continue to explore brain-related enigmas, studies like this offer vital clues in our quest to understand the most complex organ in the human body.
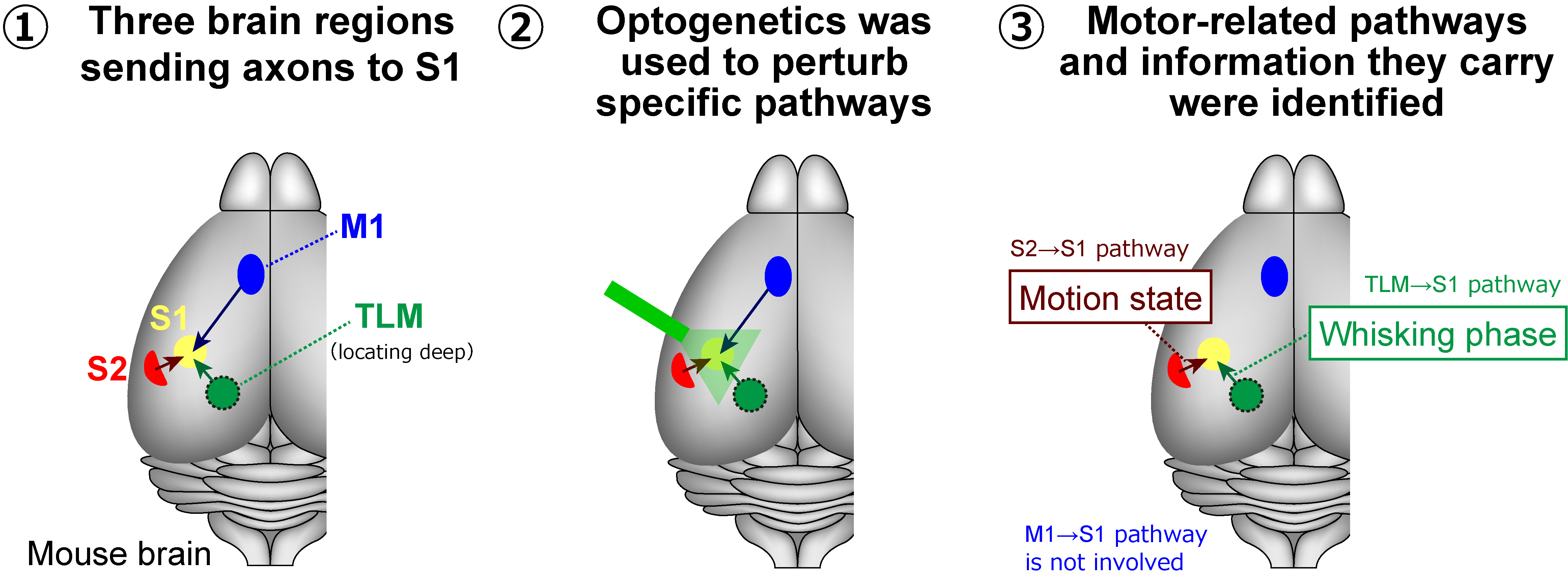 Image title: Mapping the Impact of Movement on Sensory Cortex Activity
Image title: Mapping the Impact of Movement on Sensory Cortex Activity
Image caption: Utilizing optogenetics, researchers at Fujita Health University have selectively deactivated neural pathways with light, revealing new insights into how sensory and motor areas of the mouse brain communicate during movement.
Image credit: Takayuki Yamashita from Fujita Health University
License type: Original content
Our body movements profoundly impact how our brain processes sensory information. Historically, it was believed that the brain's primary motor cortex played a key role in modulating sensory experiences during movement. However, a new study led by researchers from Fujita Health University has challenged this view. By selectively inhibiting different neural pathways in the mouse brain, they discovered that regions beyond the primary motor cortex significantly influence the primary sensory cortex during movement.
The brain is widely considered the most complex organ in the human body. The intricate mechanisms through which it processes sensory information and how this information affects and is affected by motor control have captivated neuroscientists for more than a century. Today, thanks to advanced laboratory tools and techniques, researchers can use animal models to solve this puzzle, especially in the mouse brain.
During the 20th century, experiments with anesthetized mice proved that sensory inputs primarily define neuronal activity in the primary sensory cortices—the brain regions processing sensory information, including touch, vision, and audition. However, over the past few decades, studies involving awake mice revealed that spontaneous behavior, such as exploratory motion and movement of the whiskers called whisking, actually regulates the activity of the sensory responses in the primary sensory cortices. In other words, sensations at the neuronal level appear substantially modulated by body movements, even though the corresponding neuronal circuits and the underlying mechanisms are not fully understood.
To address this knowledge gap, a research team from Japan investigated the primary somatosensory barrel cortex (S1)—a region of the mouse brain that handles tactile input from the whiskers. Their latest study, published in The Journal of Neuroscience on December 1, 2023, was conducted by Professor Takayuki Yamashita from Fujita Health University (FHU) and Dr. Masahiro Kawatani, affiliated with FHU and Nagoya University, along with their team.
The S1 region receives input through the axons from several other areas, including the secondary somatosensory cortex (S2), the primary motor cortex (M1), and the sensory thalamus (TLM). To investigate how these regions modulate activity in S1, the researchers turned to optogenetics (a technique for controlling activities of specific neuronal populations by light) involving eOPN3, which is a recently discovered light-sensitive protein enabling effective inhibition of specific neural pathways in response to light. Using viruses as a vector, they introduced the gene coding for this protein into the M1, S2, and TLM regions in mice. Then, they measured neural activity in S1 in awake mice performing spontaneous whisking. During this process, they selectively inhibited different signal inputs going to S1 using light as an ON/OFF switch and observed the effect at S1.
Interestingly, only signal inputs from S2 and TLM to S1, not from M1 to S1, modulated neuronal activity in S1 during spontaneous whisking. Specifically, the pathway from S2 to S1 seems to convey information about the motion state of the whiskers. Additionally, the TLM-to-S1 pathway appeared to relay information related to the phase of spontaneous whisking, which follows a repetitive and rhythmic pattern. These results challenge the established view that neuronal activity in sensory cortices is modulated primarily by motor cortices during movement, as Prof. Yamashita remarks: “Our findings provoke a reconsideration of the role of motor-sensory projections in sensorimotor integration and bring to light a new function for S2-to-S1 projections.”
A better understanding of how distinct brain regions modulate activities among each other in response to movement could lead to progress in myriad applied fields. These research insights have far-reaching implications, potentially revolutionizing fields like artificial intelligence (AI), prosthetics, and brain-computer interfaces. "Understanding these neural mechanisms could greatly enhance the development of AI systems that mimic human sensory-motor integration and aid in creating more intuitive prosthetics and interfaces for those with disabilities," Prof. Yamashita adds.
In summary, this study sheds light on the intricate workings of the brain. It also paves the way for researching the connection between body motion and sensory perception. As we continue to explore brain-related enigmas, studies like this offer vital clues in our quest to understand the most complex organ in the human body.

Image caption: Utilizing optogenetics, researchers at Fujita Health University have selectively deactivated neural pathways with light, revealing new insights into how sensory and motor areas of the mouse brain communicate during movement.
Image credit: Takayuki Yamashita from Fujita Health University
License type: Original content
Reference
Title of original paper
Interareal synaptic inputs underlying whisking-related activity in the primary somatosensory barrel cortexJournal
The Journal of NeuroscienceDOI
10.1523/JNEUROSCI.1148-23.2023About Professor Takayuki Yamashita
Prof. Takayuki Yamashita has been working as a professor at Fujita Health University since 2020, where he leads a laboratory within the Division of Neurophysiology. He graduated from the Faculty of Agriculture at the University of Tokyo in 2001 and completed his Ph.D. from the Graduate School of Medicine, University of Tokyo in 2007. His research team uses mice to study the neural mechanisms underlying animal behavior based on sensory preferences, applying electrophysiological measurements and optogenetics. He has contributed to over 20 research publications on these research areas.
Funding information
This work was supported by JST FOREST Program (JPMJFR204H), JSPS KAKENHI grants (21H00215, 21K19315, 23H04366, and 23H04685), The Naito Foundation, Takeda Science Foundation, Research Foundation for the Electrotechnology of Chubu, Fujita Health University and the Nagoya University CIBoG WISE program of MEXT.
