News & Topics
- May 19, 2025
- April 1, 2025
Introduction
In the Department of Disease System Analysis Medicine, we conduct basic research primarily using cultured cells and small animals such as mice. After creating various disease models using cultured cells and animals, we investigate disease mechanisms, establish diagnostic methods, and develop novel treatments, with a focus on translational research for clinical applications. We also collaborate closely with the Department of Joint Research Laboratory of Clinical Medicine at the School of Medicine and the Department of Clinical Laboratory at Fujita Health University Hospital to conduct basic and clinical research aimed at generating new insights. Undergraduate students, graduate students, and our group members actively engage in research with autonomy and conduct weekly research meetings. We provide guidance to undergraduate and graduate students to help them acquire basic research methods and skills to solve any problem.
Members
Faculty members
Medical Technology
-
 Hiroyasu Ito<br />(Professor)
Hiroyasu Ito<br />(Professor)
-
 Koji Ohashi
Koji Ohashi
(Professor) -
 Sei Saitoh
Sei Saitoh
(Associate Professor) -
 Masato Hoshi
Masato Hoshi
(Associate Professor) -
 Moeka Tanabe
Moeka Tanabe
(Assistant Profrssor)
Graduate student
Undergraduate course (SRP): Haruna Nakamura
Research Theme
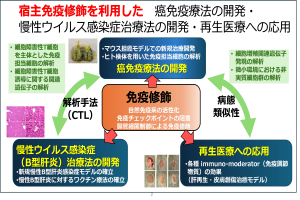
The involvement of the host immune system is essential in various diseases. Recently, there has been particular focus on the development of cancer immunotherapy and its application in clinical practice. We conduct basic research using small animals, focusing on immunology as a central theme. Our studies cover cancer (particularly the development of immunotherapies), chronic viral infections (especially hepatitis B virus), and tissue regeneration (mainly of the liver and skin). We activate the host immune system and regulate immune suppressor molecules to develop new treatments for cancer, chronic viral infections, and tissue regeneration.
In addition to collaborating with various laboratories at Fujita Health University, we also engage in joint research with Nagoya University and the University of Tokyo.(Hiroyasu Ito)
・The role of tryptophan and glucose metabolism in immune cells
・Establishment of novel biomarkers in chronic kidney disease
・The effects of rare sugars in various inflammatory diseases
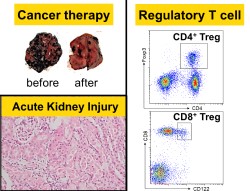
Our research interests range from cancer treatment to kidney disease. As basic research, we focus on the metabolism of immune cells using various mouse models of inflammatory diseases. In particular, the recent advent of immune checkpoint inhibitors (ICIs) has dramatically improved cancer therapy, but many patients are refractory to ICIs. We aim to solve this problem. We also aim to establish biomarkers for diagnosis and prognosis by elucidating the pathogenesis of various renal diseases, including acute kidney injury, using clinical specimens and mouse models. Although urine sample can be collected without invasion, it contains many unknown components. Our goal is to contribute to kidney disease, which is one of the most common diseases in Japan. All of our research is closely linked to clinical practice, and we are working hard every day to apply our findings to humans. (Masato Hoshi)
・Proteomics analysis of IgA nephropathy using laser microdissection
・Analysis of hepatocyte in high-fat diet mouse using wide-field observation with SEM

• Proteomics Analysis of IgA Nephropathy Using Laser Microdissection
IgA nephropathy (IgAN) is the most common type of glomerulonephritis worldwide, and effective methods for predicting disease progression are under investigation (Barbour SJ et al., JAMA Intern Med. 2019). Approximately 40% of patients develop end-stage renal failure within 20 years.
IgAN is not caused by a single genetic abnormality, but rather by a combination of multiple disease-susceptibility genes and exposure to exogenous antigens, such as upper respiratory tract infections, which can trigger acute exacerbations.
The "multi-hit mechanism" has been proposed as a pathophysiological hypothesis for IgAN (Suzuki H. et al., JASN 2011; Novak J. et al., Kidney Disease 2015). A characteristic feature of IgAN is the formation of "glycan-abnormal IgA1" and autoantibodies that react with it.
However, nephritis does not occur solely due to glycan-abnormal IgA1; multiple pathogenic "hits" are necessary for disease onset. In the transition from Hit 3 to Hit 4 in the multi-hit mechanism, it is believed that immune complexes containing glycan-abnormal IgA1 are deposited in the glomerulus, activating mesangial cells and inducing morphological changes that damage the glomerular basement membrane.
We are conducting proteomic analyses of glomeruli extracted from the kidney tissue of IgA nephropathy patients using laser microdissection, aiming to elucidate the underlying etiology of IgAN.
• Analysis of Hepatocytes in High-Fat Diet Mice Using SEM for Wide-Field Observation
We are currently observing liver tissue from high-fat diet mice using scanning electron microscopy (SEM) for wide-field analysis. High-fat diet mice develop fatty liver disease, which leads to the accumulation of fat droplets.
By administering diabetes medications to these mice, we are analyzing drug-induced changes in liver cells at the ultrastructural level.
(Sei Saitoh)
・Elucidation of the mechanisms underlying colorectal cancer in response to stress
・Development of novel anti cancer drugs targeting the gut microbiome
・Application of prebiotics to modulate the gut microbiome and prevent diseases
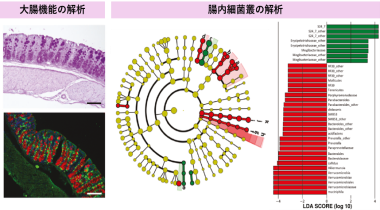
We investigate the influence of stress and nutritional factors on various diseases, focusing on the interaction between the gut microbiome and the host. In particular, we aim to elucidate how stress influences colorectal cancer and psychiatric disorders through the intestinal environment and immune response. Based on these mechanisms, we aim to the development of novel therapeutic drugs. In addition, we are investigating the roles of dietary components such as prebiotics in disease prevention through modulating gut microbiota. Through this research, we aim to establish new strategies for disease prevention and treatment by targeting the intestinal environment.(Moeka Tanabe)
Annual Events
Paper Abstracts and Progress Reports
We hold weekly meetings where department members and graduate students present their research papers and report on their research progress.
Photo Gallery
-
Coming soon…
Academic Activities
Manuscripts
2025
- Tanabe M, Kunisawa K, Saito I, Ojika H, Saito K, Nabeshima T, Mouri A. High-Cellulose DietAmeliorates Cognitive Impairment by Modulating Gut Microbiota and Metabolic Pathways inMice. J Nutr. 2025 Jun;155(6):1689-1699.
2024
2023
- Saitoh S, Takaki T, Nakajima K, Wo B, Terashima H, Shimo S, Nguyen HB, Thai TQ, KumamotoK, Kunisawa K, Nagao S, Tojo A, Ohno N, Takahashi K. Treatment of tubular damage in high-fat-diet-fed obese mice using sodium-glucose co-transporter inhibitors. PloS one.2023. 18(2)e0281770.
2022
- Yoshimura T, Yamagishi S, Akimoto Y, Saitoh S. Editorial: Brain Imaging for Glycobiology. Frontiers in Neuroanatomy. 2022. 16:1026499
2021
- Nanaka Morita, Masato Hoshi, Takeshi Hara, Soranobu Ninomiya, Taisuke Enoki, Misao Yoneda, Hisashi Tsurumi and Kuniaki Saito. Viability of diffuse large B-cell lymphoma cells is regulated by kynurenine 3-monooxygenase activity. Oncology letters. 2021 Nov;22(5):790. .
- Masato Hoshi, Hisako Kubo, Tatsuya Ando, Chieko Tashita, Kentaro Nakamoto, Yasuko Yamamoto, Hiroyuki Tezuka, Kuniaki Saito. 3-Hydroxykynurenine Regulates Lipopolysaccharide-Stimulated IL-6 Production and Protects against Endotoxic Shock in Mice. ImmunoHorizons. 2021 5(6):523-534.
- Yuko Mori, Akihiro Mouri, Kazuo Kunisawa, Mami Hirakawa, Hisayoshi Kubota, Aika Kosuge, Moe Niijima, Masaya Hasegawa, Hitomi Kurahashi, Reiko Murakami, Masato Hoshi, Takashi Nakano, Suwako Fujigaki, Hidetsugu Fujigaki, Yasuko Yamamoto, Toshitaka Nabeshima, Kuniaki Saito. Kynurenine 3-monooxygenase deficiency induces depression-like behavior via enhanced antagonism of α7 nicotinic acetylcholine receptors by kynurenic acid. Behavioural brain research. 2021, 405:113191-113191.
Access
- Access to Fujita Health University ⇒ Fujita Health University Homepage
- Access to Disease Systems Analysis Medicine 214, 3rd ⇒ University Building 3
