News & Topics
- October 1, 2025
- September 5, 2025
- April 1, 2025
Maryam joined our group as a PhD student.
Mr. Igakura received the Encouragement Award at the 21st “Wakate-no-chikara” forum.
Six undergraduate students joined our group.
Introduction
We offer laboratory research opportunities of senior thesis to undergraduate/graduate students of School of Medical Sciences, both in Faculties of Medical Technology and Radiological Technology. Research will be performed using a variety of principles in biochemistry, molecular biology, cell biology, immunology etc. Our goal in education is to develop human resources who can actively work in various research fields; not limited to hospital laboratory engineers or radiological technologists. Graduate students will be trained not only to design and conduct research but also to present/discuss their research as independent researchers.
Members
Faculty members
-
 Hiromu Takematsu
Hiromu Takematsu
(Professor)
-
 Yuko Naito-Matsui
Yuko Naito-Matsui
(Senior Assistant Professor)
Technical Staff
Graduate student
Undergraduate course
Research Theme
・Molecular functions of glycan-mediated cellular regulation
The cell surface is covered with glycans. Glycan structures are diverse depending on cell type, cellular differentiation, activation etc.; thus, glycans are often called as “the face of cells”. Glycans show various physiological functions by interacting with glycan-binding molecules (lectins) that recognize specific glycan structures.
1. Biological functions of sialic acids
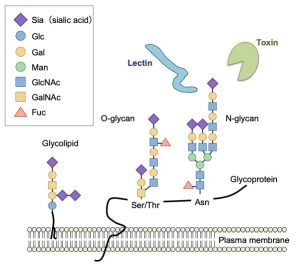 Sialoglycans
Sialoglycans
The non-reducing terminus of cell surface glycans are often occupied with sialic acids, nine-carbon acidic sugars that have negative charge. Sialic acids play pivotal roles in various cellular recognition events because of their location and structural diversity by various modification. We are studying functional significance of sialic acid-mediated molecular recognition especially focusing on immune system.
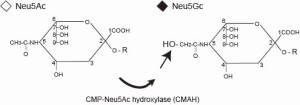 Structure of sialic acids
Structure of sialic acids
Sialic acids exhibit very specific expression patterns in mammals. Humans specifically lack the activity of CMP-Neu5Ac hydroxylase that catalyzes the biosynthesis of Neu5Gc, one of the two major sialic acid species in mammals. However, human uptakes Neu5Gc by eating Neu5Gc-containing food, such as red meat. Then, non-self Neu5Gc is expressed on the cell surface as ”xeno- autoantigen”, a uniquely acquired self-antigen. Moreover, Neu5Gc is antigenic in human immune system, that is, human has anti-Neu5Gc antibodies. As a result, Neu5Gc is reported to be a target for chronic autoimmunity. To understand immunological impact of this human-specific reaction to xeno-autoantigen for inflammatory diseases, we are trying to establish human-model mice using Neu5Gc-lacking mice.
2. Physiological significance of germinal center-specific glycan expression
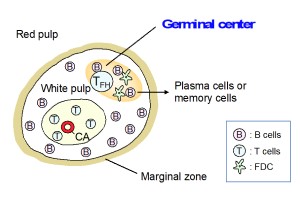 Germinal center
Germinal center
The germinal center is a microenvironment essential in acquired immune response to produce high-affinity antibodies. Neu5Gc is the major sialic acid species in mouse resting lymphocytes. We revealed that Neu5Gc expression is suppressed in B cells after the activation by antigens. This Neu5Gc suppression means the decrease in ligand for CD22 that negatively regulates B cell activation.
GL7 is a monoclonal antibody specifically staining the germinal centers, although its epitope was unknown. We found that GL7 recognizes the activation-dependent Neu5Ac induction (=decrease in Neu5Gc) in mouse lymphocyte (Neu5Gc is also suppressed in activated T cells). In addition to GL7, several antibodies or lectins recognizing specific glycan structures have been used as markers for the germinal centers. We are studying novel glycan functionalities in germinal centers as drastic changes in glycan structure occur in the site for immunological reasons.
・Molecular functions of sphingolipid-mediated signal transduction
Sphingolipids are one of the cellular membrane components. Sphingolipids have diverse molecular species and resultant cellular functionalities. We are trying to understand novel functionalities of sphingolipids to transmit cellular signaling.
1. Novel mechanism to selectively degrade sphingolipid-mediated protein kinase
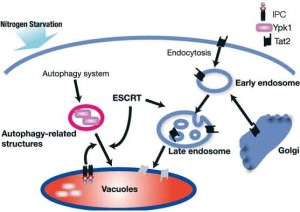 Ypk1 degradation
Ypk1 degradation
ISP-1/myriocin is a new type of immunosuppressant isolated from the entomopathogenic fungus Isalia sinclairii. The structure of ISP-1 resembles to that of sphingosine and ISP-1 was revealed to inhibit the biosynthesis of sphingolipids. We identified YPK1, encoding an AGC family serine/threonine-protein kinase to control protein translation, as one of the components of sphingolipid signaling genes in yeast. This sphingolipid-regulating protein kinase expression is controlled by the nutrient status of cells. Ypk1 is selectively degraded under nitrogen starvation. This seemed to be the case that nutrient-spending Ypk1 is degraded in advance to the bulk protein degradation of autophagy. Ypk1 degradation required specific transportation to vacuoles. We are currently analyzing the molecular mechanism in such intracellular transport system.
2. Molecular mechanism to modulate endomitosis via lyso-glycosphingolipids
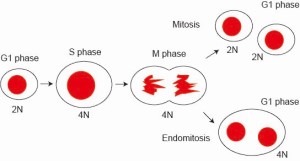 Endomitosis
Endomitosis
Lyso-sphingolipids are sphingolipids with sphingosine (not conventional ceramide) and hydrophilic head group. Psychosine is a lyso-galactosylceramide, consists of a galactose and a sphingosine. We found that psychosine has an activity to convert otherwise mitotic cells to get into “endomitotic” cell cycle, which skips cytokinesis of M-phase of cell division. Endomitosis is utilized for production of big multiploid cells such as megakaryocytes, precursor cells to platelets. We are currently trying to identify cellular components, which we call “endomitosis module”, required for psychosine-triggered endomitosis, to ultimately understand molecular mechanism of this giant-cell producing cell division.
Annual Events
Research progress meeting & Journal club
We have lab meeting once a week. Lab staffs and graduate students present their progress in research project. In journal club, we discuss recently published articles.
Presentation at academic conferences
Lab staffs and graduate students present their research results at domestic and international conferences.
Recreation
We have seasonable events such as welcome party, farewell party, after exam party, year-end party, laboratory trip etc., although these events are currently cancelled because of COVID-19.
Photo Gallery
-
 2025 Graduation ceremony
2025 Graduation ceremony
-
 2024 Graduation ceremony
2024 Graduation ceremony
-

2024 Graduation ceremony (Grad. School)
-
 2023 Graduation ceremony
2023 Graduation ceremony
Academic Activities
Manuscripts
2024
- Akatsu C, Naito-Matsui Y, Abdu-Allah HHM, Imamura A, Long W, Ishida H, Takematsu H, Tsubata T. Neu5Gc-mediated high-affinity interaction is dispensable for CD22 cis-ligands to regulate B cell signaling. J Biol Chem. 2024 Sep;300(9):107630.
2023
- Suganuma Y, Imamura A, Ando H, Kiso M, Takematsu H, Tsubata T, Ishida H. Improved synthesis of CD22-binding sialosides and its application for further development of potent CD22 inhibitors. Glycoconj J. 2023 Apr;40(2):225-246.
- Takada M, Fukuhara D, Takiura T, Nishibori Y, Kotani M, Kiuchi Z, Kudo A, Beltcheva O, Ito-Nitta N, Nitta KR, Kimura T, Suehiro JI, Katada T, Takematsu H, Yan K. Involvement of GLCCI1 in mouse spermatogenesis. FASEB J. 2023 Jan;37(1):e22680.
2022
- Hada I, Shimizu A, Takematsu H, Nishibori Y, Kimura T, Fukutomi T, Kudo A, Ito-Nitta N, Kiuchi Z, Patrakka J, Mikami N, Leclerc S, Akimoto Y, Hirayama Y, Mori S, Takano T, Yan K. A Novel Mouse Model of Idiopathic Nephrotic Syndrome Induced by Immunization with the Podocyte Protein Crb2. J Am Soc Nephrol. 2022 Nov;33(11):2008-2025.
- Doi H, Matsui T, Dijkstra JM, Ogasawara A, Higashimoto Y, Imamura S, Ohye T, Takematsu H, Katsuda I, Akiyama H. Andrographolide, isolated from Andrographis paniculata, induces apoptosis in monocytic leukemia and multiple myeloma cells via augmentation of reactive oxygen species production. F1000Res. 2021 Jul 6;10:542.
- Takahashi S, Fukuhara D, Kimura T, Fukutomi T, Tanaka E, Mikami N, Hada I, Takematsu H, Nishibori Y, Akimoto Y, Kiyonari H, Abe T, Huber O, Yan K. USP40 deubiquitinates HINT1 and stabilizes p53 in podocyte damage. Biochem Biophys Res Commun. 2022 Jul 23;614:198-206.
- Enterina JR, Sarkar S, Streith L, Jung J, Arlian BM, Meyer SJ, Takematsu H, Xiao C, Baldwin TA, Nitschke L, Shlomchick MJ, Paulson JC, Macauley MS. Coordinated changes in glycosylation regulate the germinal center through CD22. Cell Rep. 2022 Mar 15;38(11):110512.
- Akatsu C, Alborzian Deh Sheikh A, Matsubara N, Takematsu H, Schweizer A, Abdu-Allah HHM, Tedder TF, Nitschke L, Ishida H, Tsubata T. Chondroitin 6-sulphate is required for neuroplasticity and memory in ageing. Sci Signal. 2022 Mar;15(723):eabf9570.
Conferences
2024
- Motoki Igakura, Yuko Naito-Matsui, Hiromu Takematsu. ヒト特異的な異種自己抗原免疫応答状態のマウスモデル構築に向けた抗Neu5Gcモノクローナル抗体の樹立. 糖鎖科学中部拠点 20th “Wakate-no-chikara” forum (September, poster)
- Taishi Yuasa, Yuko Naito-Matsui, Hiromu Takematsu. CD77発現により変化するCD19上のN型糖鎖構造の解析. 糖鎖科学中部拠点 20th “Wakate-no-chikara” forum (September, poster)
- Motoki Igakura, Yuko Naito-Matsui, Hiromu Takematsu. ヒト特異的な異種自己抗原免疫応答状態のマウスモデル構築に向けた抗Neu5Gcモノクローナル抗体の樹立. The 43rd Annual Meeting of Japanese Society of Carbohydrate Research (September, poster)
- Taishi Yuasa, Yuko Naito-Matsui, Hiromu Takematsu. CD77発現により変化するCD19上のN型糖鎖構造の解析. The 43rd Annual Meeting of Japanese Society of Carbohydrate Research (September, poster)
- Hiromu Takematsu, Yuko Naito, Yu Tanaka, Kiyoko F. Aoki-Kinoshita "CIRES database to explore biosynthetic pathway enzyme(s)" Glyco-core Symposium 2024 (July, poster)
- Yuko Naito-Matsui, Hikari Asano, Kumiko Hamano-Takada, Takeshi Tsubata, Shogo Oka, Hiromu Takematsu "PNA epitopes expressed on germinal center B cells modulate B cell receptor signaling" Glyco-core Symposium 2024 (July, poster)
- Taishi Yuasa, Naito-Matsui Yuko, Hiromu Takematsu "Globotriaosylceramide/CD77 enhances N-glycosylation on CD19 and attenuates B cell receptor signaling" Glyco-core Symposium 2024 (July, poster)
2023
- Motoki Igakura, Yumina Sato, Yuki Yamato, Yuko Naito-Matsui, Hiromu Takematsu. ヒト特異的な異種自己抗原Neu5Gcに対する免疫応答マウスモデル樹立に向けた基盤的研究. 糖鎖科学中部拠点 19th “Wakate-no-chikara” forum (September, oral)
- Taishi Yuasa, Yuko Naito-Matsui, Hiromu Takematsu. スフィンゴ糖脂質CD77発現によるCD19上のN型糖鎖変化はBCRシグナル伝達を抑制する. The 42nd Annual Meeting of Japanese Society of Carbohydrate Research (September, oral)
- Motoki Igakura, Yumina Sato, Yuki Yamato, Yuko Naito-Matsui, Hiromu Takematsu. ヒト特異的な異種自己抗原Neu5Gcに対する免疫応答マウスモデルの樹立にむけた基盤的研究. The 42nd Annual Meeting of Japanese Society of Carbohydrate Research (September, oral)
- Taishi Yuasa, Yuko Naito-Matsui, Hiromu Takematsu. スフィンゴ糖脂質CD77の発現によるCD19上のN型糖鎖の変化はB細胞抗原受容体シグナル伝達の抑制に関わる. 糖鎖科学中部拠点 18th “Wakate-no-chikara” forum (January, oral)
2025
- Yuko Naito-Matsui, Kazuya Inoue, Haruka Tanaka, Kasumi Nishigaki, Kiyoko F. Kinoshita-Aoki, Hiromu Takematsu "Development of CIRES database that enables to identify glycan biosynthesis-regulating enzyme genes" Glyco-core Symposium 2025 (September, poster)
- Motoki Igakura, Yuko Naito-Matsui, Hiromu Takematsu "Development of Anti-Neu5Gc Antibodies to Establish a Mouse Model for Human Inflammation" Glyco-core Symposium 2025 (September, poster)
- Taishi Yuasa, Naito-Matsui Yuko, Hiromu Takematsu "Germinal center B cell marker CD77 regulates B cell receptor signaling via CD19" Glyco-core Symposium 2025 (September, poster)
- Motoki Igakura, Yuko Naito-Matsui, Hiromu Takematsu. α2-3, α2-6, α2-8結合Neu5Gcを特異的に認識する抗Neu5Gcモノクローナル抗体の樹立. 糖鎖科学中部拠点 21st “Wakate-no-chikara” forum (September, oral)
Review
- Yuko Naito-Matsui. Sialoglycan-modified mice to evaluate evolutional effects. SEIKAGAKU. 2020; 92(2):263-267. (Japanese)
Access
- Access to Fujita Health University ⇒ Fujita Health University Homepage
- Access to Molecular Cell Biology ⇒ Room 332, University Building 3

